Atrio-Ventricular Abnormalities (WPW) Ablation
The Atrio-Ventricular Abnormalities (Wolff-Parkinson-White syndrome WPW) Ablation consists of administering thermal energy (radio frequency) near the accessory pathway in order to create irreversible cell damage and therefore make it electrically inert.
Indice dell'articolo
What is Wolff-Parkinson-White syndrome (WPW)?
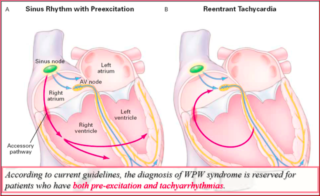
Wolff-Parkinson-White syndrome (WPW) is characterized by the association of symptoms due to tachyarrhythmias with the presence of pre-excitation on the surface ECG tracing (WPW pattern). In WPW syndrome, tachyarrhythmias are due to a phenomenon of atrio-ventricular macro-re-entry, which recognizes two anatomically defined conduction pathways: The hisian node system and the atrio-ventricular accessory pathway itself. It is sufficient that between these two ways there is a difference in the refractory period or in the conduction speed for a return circuit to be created. Atrio-ventricular re-entry tachycardias (AVRT) are commonly distinguished in orthodromic and antidromic depending on whether the anterograde conduction occurs through the node-hisian system or through the accessory pathway.
What is the role of the electrophysiological study in patients with WPW?
The electrophysiological study in patients with WPW is useful to confirm the diagnosis, study the mode of initiation of tachycardias, locate the accessory pathways, demonstrate that the accessory pathway participates in tachycardias, evaluate the refractory nature of the accessory pathway and its implications for the risk of dangerous arrhythmias, stop tachycardias in drug therapies, and stimulate or ablate arrhythmias associated with WPW syndrome.
The episodes of recurrent supraventricular tachycardia (SVT) can start from early childhood, but their onset is more frequent during adolescence or adulthood. Paroxysmal atrial fibrillation, on the other hand, appears almost exclusively in adults. It is not uncommon to see signs of pre-excitation ECG at birth, which disappear after some time, as the accessory pathway degenerates and becomes fibrotic, becoming unable to conduct; however the disappearance of the pre-excitation signs of the ECG does not necessarily mean that there is a complete elimination of the conduction on the Kent beam, since both the latent and hidden pre-excitation do not associate with the typical ECG scheme (delta wave, short interval PR, etc.) but both are capable of inducing arrhythmias (3).
An extremely low percentage of patients with WPW suddenly die from ventricular fibrillation. The mechanism is almost certainly an atrial fibrillation with a high ventricular response, which degenerates into a ventricular fibrillation due to the high ventricular rate. This is a dramatic event that can also occur in asymptomatic subjects, with an incidence of 1:1000 people per year. It was observed that all patients with pre-excitation who were revived from cardiac arrest had a short antegrade refractory period (< 250 ms) of the accessory pathway. On the basis of these data it has been proposed to consider patients “at risk” with this electrophysiological result (4). However, the positive predictive value of this parameter is very low, since about 20% of the subjects subjected to an EP study have these characteristics and should therefore be considered at risk, while the actual incidence of sudden death is considerably lower.
What is the role of endocardial mapping in WPW?
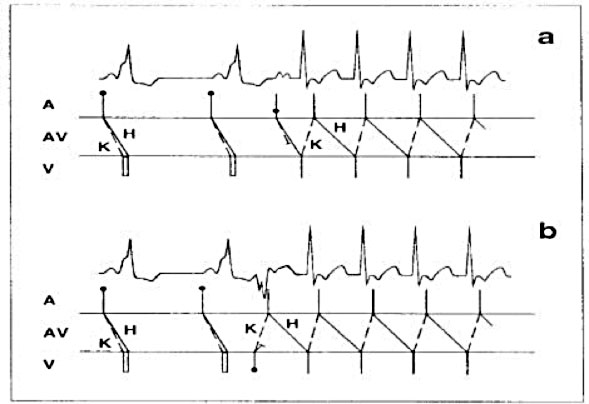
The ECG can help locate the accessory pathways (AP), while the electrophysiological study and intracavity mapping provide precise data on its position and on the electrophysiological properties of the accessory pathways. In order to define the exact position of the Kent beam, the AV annulus is mapped in order to find the point with the shortest AV interval during the antegrade conduction on the Kent beam or the shortest AV interval during the ventricular pacing or orthodromic tachycardia. This type of mapping can be performed using bipolar or unipolar recordings and is based on the principle that the activation of the first chamber (ventricular during antegrade, atrial conduction during retrograde conduction) allows to locate the insertion of the accessory pathway in the chamber. Therefore, the scaler catheter must be positioned on the right or left AV ring, in contact with the endocardium, and then moved until the shortest conduction interval is found. The position of the catheter is confirmed by fluoroscopy and the recorded potential, which is composed of two deflections, the atrial and ventricular ones. If the catheter is on the Kent beam, it is easy to record almost fused A and V waves, indicating an extremely short conduction time. Sometimes it is even possible to record the potential of the Kent beam, seen as a rapid short-term deflection, between A and V, expressing the depolarization of the accessory pathway: the waves A and V and the Kent potential are continuous, and the different components are difficult to separate. The identification of this continuous electrical activity strongly indicates the presence of an accessory route.
Antegrade mapping
The first ventricular activation site during manifest pre-excitation (pre-excited sinus rhythm, antidromic AVRT) identifies the site of ventricular insertion of the accessory pathway. Target site criteria for ablation during antegrade mapping include: 1) AP potential (Kent potential), 2) first local ventricular activation related to the onset of the delta wave (pre-delta) and 3) fusion of atrial and ventricular electrograms. The potentials of the accessory path reflect a rapid local activation of the accessory pathway and are acute and high frequency deflections between the atrial and ventricular electrograms that precede the onset of the delta wave. The more the local ventricular electrogram on the ablation catheter precedes the onset of the delta wave, the greater the probability of success.
Retrograde mapping
The first atrial activation site during retrograde conduction on the accessory pathway (ventricular pacing, orthodromic AVRT) identifies its atrial insertion site. A limitation of the mapping during ventricular pacing is that retrograde conduction on the AV node may interfere with the identification of the first atrial activation site on the accessory pathway (in particular, septal accessory pathways). Potential solutions include stimulation at a higher speed (to cause decrease or blockage in the AV node), administration of drugs that slow AV nodal conduction or mapping during orthodromic AVRT (where retrograde conduction occurs only on the accessory pathways). The criteria for defining the site for ablation include: 1) potentials on the accessory pathways, 2) the first atrial activation site and 3) fusion of electrograms A and V.
Based on the recommendations of the ACC / AHA / ESC guidelines of 2019, in the case of asymptomatic pre-excitation, the execution of the EP test is recommended in the young person, in the athlete and in subjects in which the non-invasive tests suggest a non-low risk situation. In subjects with asymptomatic WPW, where the EP test with the use of isoprenaline shows high risk properties, such as SPERRI < 250 ms, AP ERP < 250 ms, multiple accessory pathways and tachycardia mediated by inducible accessory route, there is a class I indication for RF.
What are the features of atrioventricular accessory pathway-mediated tachycardias (AVRT)?
The most common tachycardias associated with WPW syndrome are circuit tachycardias, 95% of which are orthodromic; that is, they lead downwards with respect to the normal A-V conduction system and retrograde on the bypass section. The conduction and refractory relationship of the normal A-V conduction system and the bypass section, as well as the stimulation site, determine both the ability to start the tachyarrhythmia circuit, and, theoretically, the type of tachycardia. The conduction and refractory nature of the accessory pathways in most cases behave like contractile muscle tissue; therefore, the accessory pathways demonstrate rapid conduction and present refractory periods, which tend to shorten with the reduction of the lengths of the stimulation cycle (PCL).
WPW syndrome allows to verify the presence of all the requisites for a re-entering rhythm: (a) two anatomical pathways determining from the functional point of view; (b) one-way block in one of the paths (in this case, in the accessory path or in the nodal A-V path); (c) a sufficient slowdown in a part of the circuit to overcome the refractoriness before the circulating impulse; and (d) the impulse conduction time must exceed the longest effective refractory period of any component in the circuit. Both the antegrade and retrograde refractory periods of the accessory pathway are the main determinants of: (a) ability to initiate and sustain circular movement, and (b) ventricular response to atrial tachyarrhythmias (e.g. atrial fibrillation, atrial flutter, and atrial tachycardia).
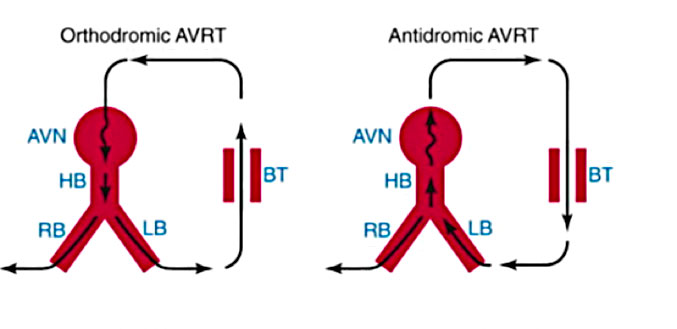
AVRT is a re-entrant arrhythmia and is classified into orthodromic and antidromic variants. During orthodromic tachycardia, the antegrade pathway is the AV-His-Purkinje node system and the retrograde pathway is the accessory pathway. On the contrary, during antidromic tachycardia, the antegrade pathway is the accessory pathway and the retrograde pathway is the normal conduction system. Orthodromic AVRT constitutes about 95% of spontaneous and laboratory-induced AVRT. For the onset of tachycardia, a premature atrial complex (APC), spontaneous or induced by stimulation, hangs on the accessory pathway and travels along the AV-His-Purkinje node. The impulse conducted reaches the ventricle and returns to the atrium on the accessory route, which has now recovered its excitability. The impulse then returns to the AV-His-Purkinje system, perpetuating tachycardia. Orthodromic tachycardia can also be initiated by a premature ventricular complex (PVC). In this case, PVC blocks the His-Purkinje system but travels on the accessory route to the atrium. If the node’s AV-His-Purkinje system has recovered excitability, the impulse then travels along the node and returns to the ventricle, and orthodromic tachycardia is initiated.
What are the goals in the treatment of WPW syndrome?
Pre-excitement therapy has four different objectives: 1. To cure symptoms; 2. Prevent the risk of sudden death; 3. Prevent or cure, in case of chronic tachycardia, the worsening of the ventricular function; 4. Allow subjects with pre-excitement to carry out all activities that are otherwise prohibited by law when there is pre-excitement on the ECG, for example in competitive sportsmen or in workers of professions at risk.
In other cases, therapy is not indicated: in particular in asymptomatic subjects, who present with only pre-excitation on the ECG, no treatment is necessary, once the absence of risk parameters in the electrophysological properties of the accessory pathways has been verified, given except that in rare cases, the risk of developing dangerous arrhythmias is very limited.
There are four different types of therapeutic approaches: antiarrhythmic drugs, transcatheter ablation of accessory pathways, surgical ablation of accessory pathways, and electrical therapy (cardioversion, stimulation).
What are the pharmacological treatments of AVRT in WPW?
In orthodromic AVRT, the AV node is the weak link, and drugs that prolong AV nodal refractoriness or depress its conduction can lead to a blockage in the node with consequent interruption of tachycardia. Vagal maneuvers end tachycardia, causing blockage in the node. First-line drugs that are effective in the acute termination of orthodromic AVRT include the administration of adenosine, verapamil, diltiazem, or beta-blockers via IV route. Digoxin is less effective due to the delayed start of the action. Among class 1a antiarrhythmic drugs, procainamide is a valid alternative, since it depresses conduction, prolongs refractoriness in most cardiac tissues (i.e. atrium, ventricle and His-Purkinje system) and also blocks conduction in the accessory pathway. Ic class antiarrhythmic drugs are more effective than class Ia drugs in blocking AP conduction; however, they should be avoided in patients with structural heart disease. Amiodarone has various electrophysiological effects but is no more effective than class IC drugs used alone or in combination with beta-blockers. In general, amiodarone should be reserved for those who are candidates refractory to drugs, elderly, or unfit for ablative therapy. Sotalol can be effective in preventing tachycardia, although it is associated with a 4% risk of torsades de pointes, especially in those with significant structural heart disease and congestive heart failure. Oral digoxin is not effective as a monotherapy for orthodromic AVRT and, due to its direct effects on the accessory pathway, this drug can actually accelerate conduction on the accessory pathway during atrial fibrillation. Therefore, digoxin should never be used to treat patients with pre-excitation.
In antidromic AVRT, retrograde AV nodal conduction can be the weak link in the re-entry circuit. Calcium channel blockers, beta blockers and adenosine can be used for the acute cessation of tachycardia. Procainamide IV is the drug of choice in the acute treatment of antithromic AVRT. Also this drug does not stop tachycardia: it can slow down the rate of tachycardia. In the absence of contraindications, class 1c drugs are the drugs of choice for the long-term oral treatment of antidromic tachycardia.
How is radiofrequency catheter ablation performed in WPW?
Radiofrequency ablation (RF) is the procedure of choice for patients with symptomatic WPW syndrome and for those who respond poorly to medical therapy. In the more experienced centers, the success rate is between 95% and 97% with a recurrence rate of 6%. The success of the ablation depends critically on the accurate location of the accessory route. The location of the preliminary route can be obtained from the delta wave and QRS morphologies (see location of accessory pathways from the surface ECG).
In general, the endocavity electrophysiological study precedes the ablation of the accessory pathway, locating its exact location. The ablation procedure is performed under local anesthesia and a mild pharmacological sedation. Multiple venous accesses (usually right femoral and left subclavian) are obtained by the Seldinger technique. If the accessory pathway has a left localization, an arterial access (right femoral artery) is also positioned in order to allow ablation by transaortic approach. Alternatively, the left heart chambers can be reached by transseptal puncture.
The quadripolar diagnostic leads are positioned at the level of the upper right atrium, the bundle of His, at the apex of the right ventricle and in the coronary sinus (cardiac vein that surrounds the left ventricular atrium sulcus and allows to record the electrical activity in the left part of the heart).
The ablation consists of administering thermal energy (radio frequency) near the accessory pathway in order to create irreversible cell damage and therefore make it electrically inert.
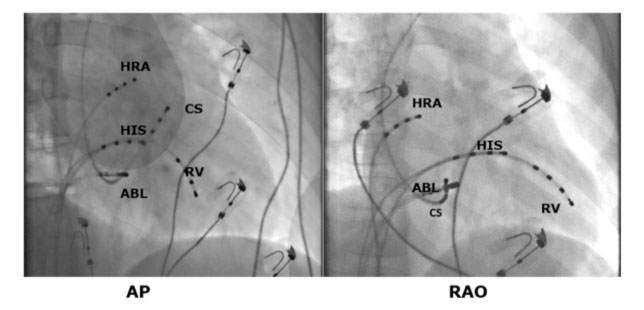
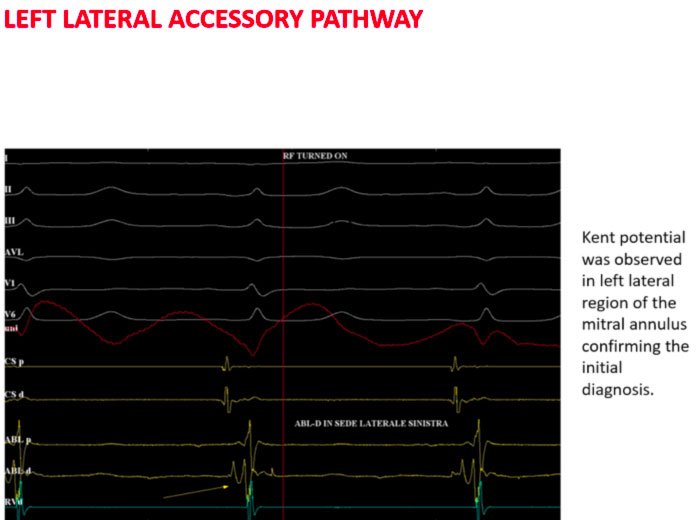
If the ventricular excitation occurs during the electrophysiological study, the valve rings are mapped, and it is identified where the earliest ventricular activation or the characteristic Kent potential is present.
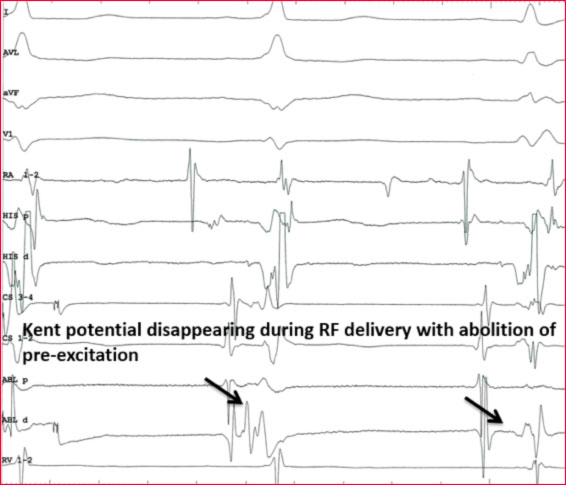
Some atrial or ventricular pacing maneuvers can be helpful if the accessory pathway is not immediately identifiable.
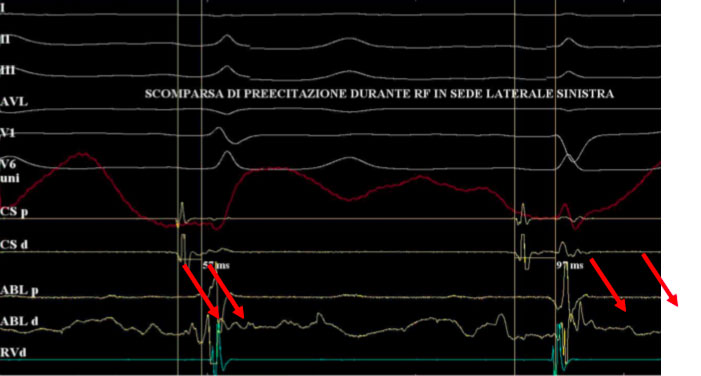
When pre-excitation is not maximum, rapid atrial stimulation or adenosine I.V. can be used to obtain complete pre-excitation in order to improve the accuracy of the location. This is particularly useful in the left lateral accessory pathways where pre-excitation can be improved with left atrial stimulation (from the coronary sinus, CS, catheter). The criteria of the intracardiac electrogram (8) used to identify the appropriate target sites for the ablation of the manifested pathways include the presence of a long potential at the accessory avia (Fig. 5), the early onset of local ventricular activation with respect to onset of the delta wave, the stability of the electrogram, and the continuous anterograde electrical activity (fused atrial and ventricular electrodes).
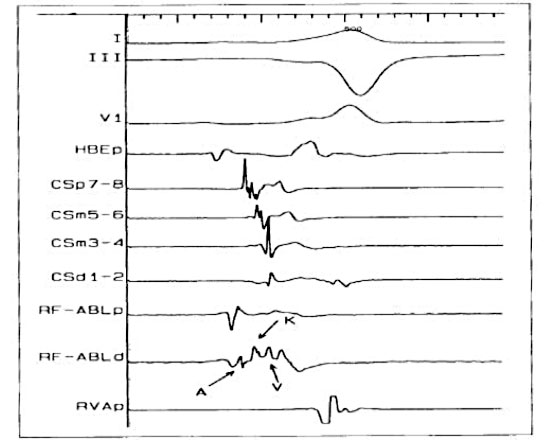
What are the criteria for choosing the approach for ATCRF of abnormal pathways?
The criteria used to identify the appropriate target sites for the ablation of the hidden pathway include the presence of retrograde potential along the accessory pathway, continuous retrograde electrical activity with ventricular stimulation or during tachycardia and electrogram stability. Ablation can be guided by the catheter in the coronary sinus used to fix the position of the path. The anomalous route can be ablated through a trans-septal or trans-aortic retrograde approach, depending on the experience and preferences of the operator. In the absence of a PFO, the trans-septal approach involves puncture through the oval fossa. With the trans-aortic approach, the tip of the ablation catheter is curved to avoid damaging the coronary arteries, advancing retrograde through the aortic valve in the left ventricle and positioned along the mitral ring.
Catheter ablation is associated with a very high success rate. The correct ablation of the paths of the free walls on the right requires a detailed mapping of the lateral tricuspid annulus. The overall success rate for ablation of the free wall of the right ventricle is the lowest of all accessory pathways, with an average of 90% and a recurrence rate of 14%. The reasons for the reduction of the success rate include the instability of the catheter and the lack of a coronary sinus-like structure on the right side, parallel to the tricuspid ring, to facilitate mapping.
Ablation of the antero-septal and median pathways can be difficult due to the proximity to the A-V node and His bundle, however it is associated with an overall success rate of 95% to 98% and a risk of 1% to 3% of permanent A-V blockade. Ablation of the posterior-septal pathways can be challenging due to the complex anatomy of the posterior-septal area. Most postero-septal pathways can be ablated from the right side, although in 20% of cases a left side approach is needed (Fig. 6).
ECG and electrophysiological aspects that suggest the need for a left-sided approach include a positive delta wave or a positive QRS complex in V1, the first retrograde atrial activation at the coronary sinus ostium (CS) and an increase in the VA interval with the presence of BBSX during orthodromic tachycardia.
A small percentage of the accessory pathways have an epicardial site. Between 5% and 17% of postero-septal and posterior APs are located at the epicardial level and ablation by CS is necessary. The presence of epicardial accessory pathways could be suggested by the discovery of small or absent potentials during endocardial mapping and larger potentials during CS level mapping. The epicardial accessory pathways on the left side can be successfully ablated within the CS in the case of routes with high potential. However, the ablation of accessory pathways in other epicardial sites may require a percutaneous epicardial approach, as an alternative to cardiac surgery.
Overall, AP ablation is associated with a complication rate of 1% to 4% and a procedure-related mortality rate of approximately 0.2%. Complication of complete A-V block occurs in about 1% of patients and is most frequently found in patients undergoing septal pathway ablation. Autonomic dysfunction and inappropriate sinus tachycardia are rare complications of radiofrequency ablation of the atrium-ventricular accessory pathways and are less frequent than those observed in AVNRT ablation. Today, advances in lead design, energy delivery systems, mapping systems, and remote robotic navigation systems have made transcatheter ablation the therapy of choice for most macro-tachycardias (ARVT and AVNRT).
Presence of multiple accessory routes.
In about 10-15% of subjects with pre-excitation, there are multiple accessory pathways. Histopathological data show a higher frequency of multiple accessory pathways than those observed clinically. The presence of multiple accessory pathways increases the incidence of symptoms and is associated with a higher risk of sudden death due to atrial fibrillation that degenerates into ventricular fibrillation. Patients with pre-excitation resurrected from sudden death had a higher incidence of multiple accessory pathways than the control group that had not had cardiac arrest (5). Diagnosis of multiple accessory pathways on the ECG is possible, although not in all cases.
EXAMPLES OF ABLATION PROCEDURES IN SPECIFIC SITUATIONS ARE SHOWN BELOW
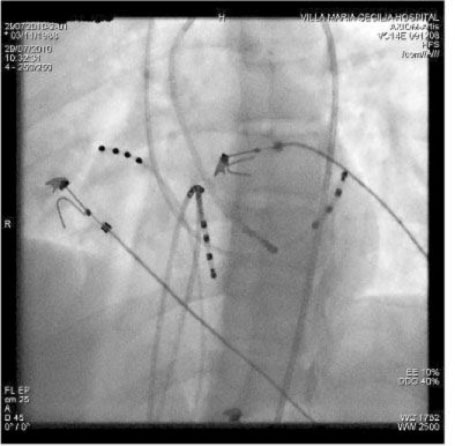
Ablation of a manifest left postero-septal AP using a retrograde trans-aortic approach (LAO projection). The ablation catheter is positioned along the postero-septal mitral annulus where it records potential on the accessory pathways between the atrial and ventricular electrograms. The application of RF energy on this site caused the loss of pre-excitation within seconds. The CS catheter provides a useful reference to the mitral ring.
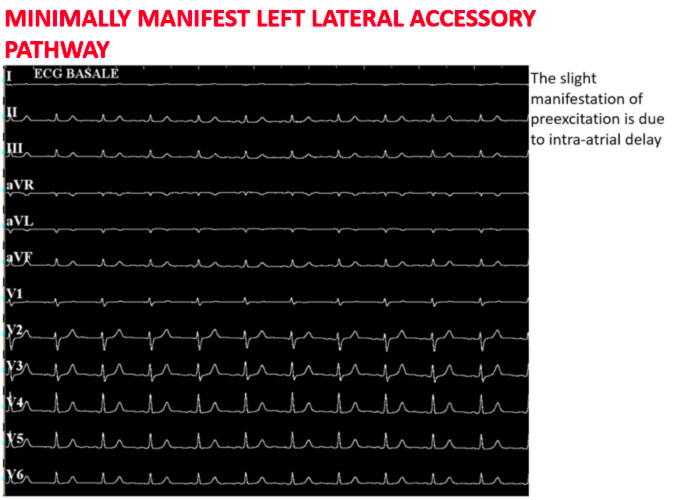
The figure above shows how in basal conditions the signs of ventricular pre-excitation are not very evident
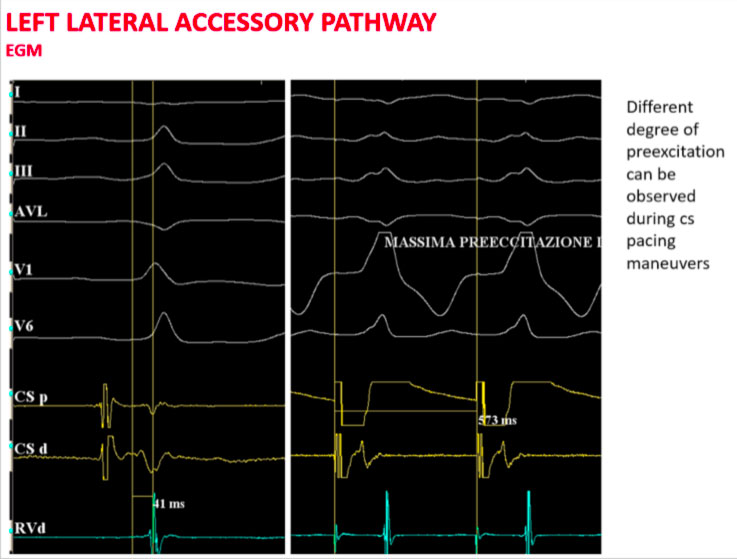
Proceeding with an asynchronous stimulation from the coronary sinus increases the degree of ventricular pre-excitation with the shortest AV interval detectable near the distal coronary sinus.
In the above case, for example, the scaler catheter is positioned trans-aortically in the left lateral position and the typical Kent potential is recorded on the distal dipole.
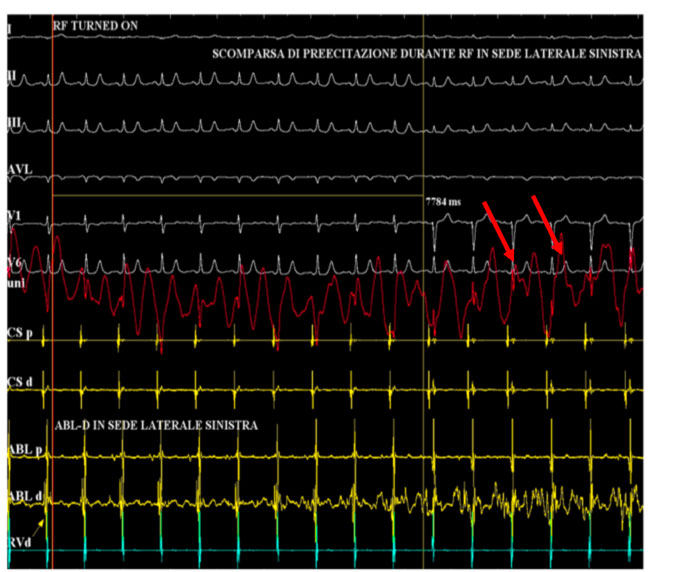
Here, radiofrequency is delivered with the disappearance of the ventricular pre-excitation.
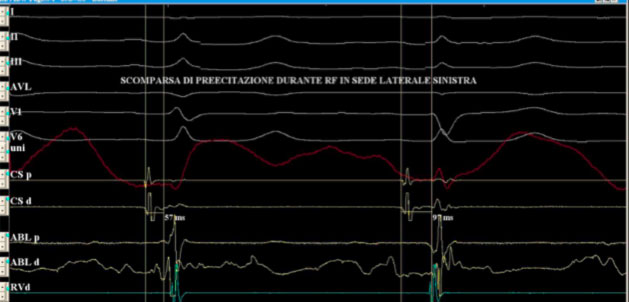
Note how the atrial and ventricular signals appear more spaced on the dipole in the coronary sinus.
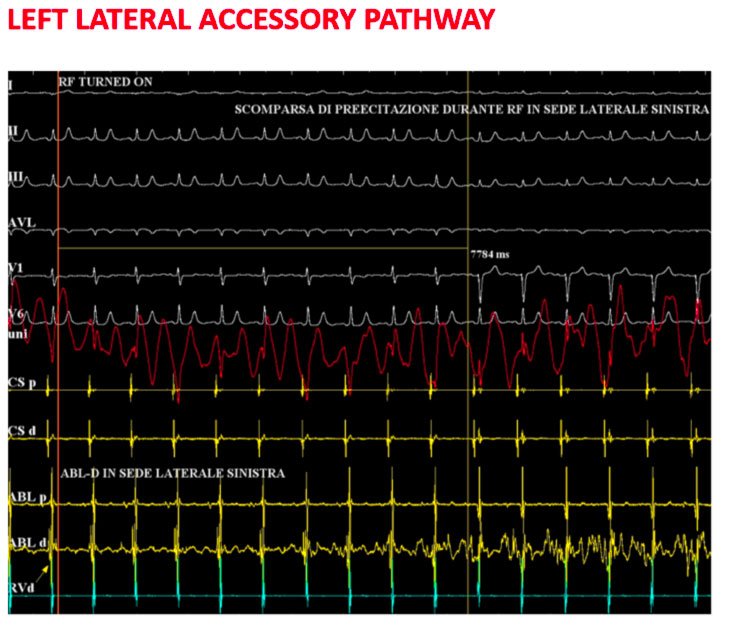
At the end of the ablation procedure, the electrocardiogram no longer shows the stigmata of the pre-excitation and therefore a complete normalization of the same is obtained.
Sometimes accessory pathways can complicate other arrhythmic diseases, such as nodal re-entry tachycardia or atrial fibrillation. Just the latter when associated with ventricular pre-excitation can constitute a real clinical emergency due to the lack of “filter” of the AV node and the high ventricular response conferred by the low refractory period of the accessory pathway.
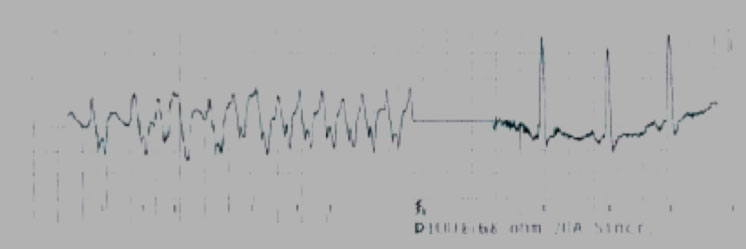
Some atrial or ventricular pacing maneuvers can be helpful if the accessory pathway is not immediately identifiable.
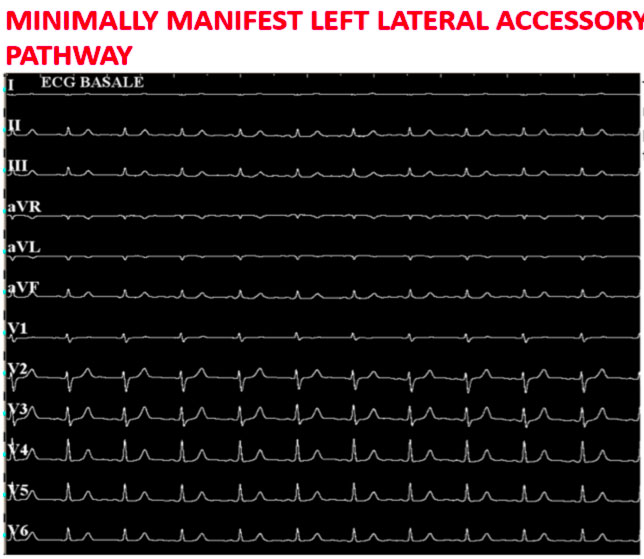
The figure above shows how in basal conditions the signs of ventricular pre-excitation are not very evident.
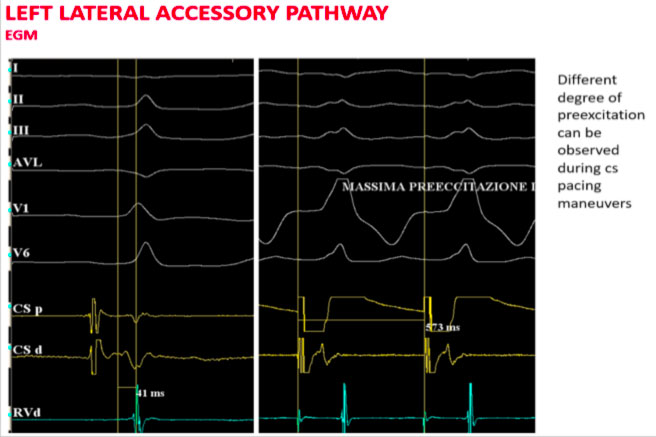
Proceeding with an asynchronous stimulation from the coronary sinus increases the degree of ventricular pre-excitation with the shortest AV interval detectable near the distal coronary sinus.
In the above case, for example, the scaler catheter is positioned trans-aortically in the left lateral position and the typical Kent potential is recorded on the distal dipole.
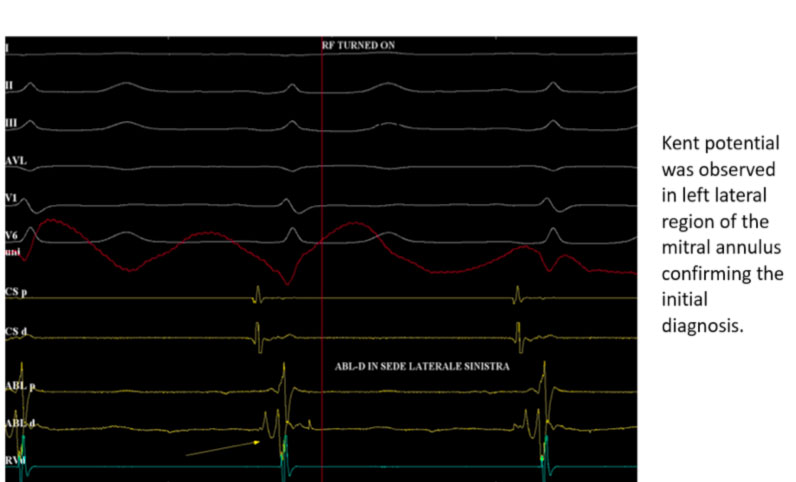
Here, radiofrequency is delivered with the disappearance of the ventricular pre-excitation.
Note how the atrial and ventricular signals appear more spaced on the dipole in the coronary sinus.
At the end of the ablation procedure, the electrocardiogram no longer shows the stigmata of the pre-excitation and therefore a complete normalization of the same is obtained.
When is transcatheter ablation recommended in asymptomatic subjects with WPW?
Currently, the importance of electrophysiological study (EP) and transcatheter ablation of accessory pathways are well established in symptomatic patients with WPW syndrome. Based on the recommendations of the 2019 ACC / AHA / ESC guidelines, in the case of symptomatic pre-excitation, transcatheter ablation has a class I indication.
The approach to asymptomatic patients is less clear. The increased safety of the EP study and catheter-based ablation techniques provide an impetus to prophylactic ablation of the pathway. To support this, randomized studies have shown that prophylactic ablation in asymptomatic patients who are at high risk of arrhythmias, performed in experienced centers, reduces the risk of life-threatening arrhythmias.
What is the role of surgical ablation in WPW?
Elective surgical treatment of WPW has been largely abandoned. Until the 1980s, several patients underwent surgery to stop conduction on the AP, but since catheter ablation became available, it has been universally accepted that the risk / benefit ratio of this surgery was unacceptable, since better results were obtained using simpler and less traumatic methods.
What is the role of electric therapy in WPW?
Electrical pre-excitation therapy is based on cardioversion, which is used in case of pre-excited atrial fibrillation and rarely for AVRT, and on atrial or ventricular stimulation in case of re-entering tachycardia. Atrial stimulation can be performed through the endocavitary or transesophageal pathway, while ventricular stimulation can only be performed through the endocavitary pathway. This type of approach is recommended in subjects where drug administration is not possible or an AVRT does not stop after vagal maneuvers and the arrhythmia is not well tolerated.
What are the risk parameters of dangerous arrhythmias in WPW?
Unlike the AV node, the accessory pathways do not demonstrate a frequency-dependent decremental conduction that slows down with faster atrial rates. The following characteristics identify low risk accessory pathways: 1) intermittent pre-excitation, 2) exercise induced blockage of the accessory pathway, 3) shorter pre-excited RR interval during AF > 250 ms and 4) loss of pre-excitation with procainamide, ajmaline or disopyramide (7). Intermittent pre-excitation demonstrates that the accessory pathway is unable to sustain 1:1 conduction during sinus rhythm, and therefore cannot conduct rapidly during AF.
Similarly, the sudden loss of pre-excitation during exercise shows that the accessory pathway is unable to sustain 1:1 conduction during exercise-induced sinus tachycardia. During exercise, the sudden loss of pre-excitation (blockage of the accessory pathway dependent on frequency) must be differentiated from the gradual loss of pre-excitation (pseudonormalization) due to a better AV nodal conduction. During pseudonormalization, the accessory pathway continues to lead anterograde, but the delta wave slowly disappears as the contribution to the ventricular activation by the AV-His-Purkinje system increases. Since the antegrade effective refractory period (ERP) is related to the shortest expected RR interval during AF, an antegrade ERP along the accessory pathway or a duration of the atrial pacing cycle maintaining a 1:1 conduction shorter than 250 ms is a reasonable, but not ideal substitute for the minimum RR interval, in cases where AF is absent.
1. Santinelli V, Radinovic A, Manguso F, Vicedomini G, Gulletta S, G Paglino, Mazzone P, Ciconte G, Sacchi S, Sala S, Pappone C. The natural history of asymptomatic ventricular preexcitation. A long-term prospective follow-up study of 184 asymptomatic children. J Am Coll Cardiol. 2009; 53: 275-280. Impact factor: 11.438 2. Santinelli V, Radinovic A, Manguso F, Vicedomini G, Ciconte G, Gulletta S, Paglino G, Sacchi S, Sala S, Ciaccio C, Pappone C. Asymptomatic ventricular preexcitation: A long-term prospective follow-up study of 293 adult patients. Circ Arrhythmia Electrophysiol 2009; 2:102-107. 3. Pappone C and Santinelli V. Remote ablation of accessory pathways. Eur Heart J.2008 Feb; 29 (3): 422. Impact Factor: 8.917 4. Pappone C, Radinovic A and Santinelli V. Sudden death and ventricular preexcitation: is necessary to treat the asymptomatic patients? Curr Pharmac Design. 2008; 14 (8) 762-765. Impact factor 4.399 5. Triedman J, Perry J, Van Hare G, Pappone C, Santinelli V. Risk Stratification for Prophylactic Ablation in Asymptomatic Wolff–Parkinson–White Sindrome. The N ew England Journal of Medicine 2005; 352(1): 92-93. Impact Factor: 50.017 6. Pappone C and Santinelli V. Catheter ablation should be performed in asymptomatic patients with Wolff-Parkinson-White syndrome. Circulation. 2005 Oct 4;112(14):2207- 2215. Impact factor: 14.595 7. Pappone C, Santinelli V, Rosanio S, Vicedomini G, Nardi S, Pappone A, Tortoriello V, Manguso F, Mazzone P, Gulletta S, Oreto G, Alfieri O. Usefulness of invasive electrophysiologic testing to stratify the risk of arrhithmic events in asymptomatic patients with Wolff-Parkinson-White Pattern. Results from a large prospective longterm follow-up.study. J Am Coll Cardiol. 2003; 41, 2: 239-244. Impact factor: 11.438