Atrial Fibrillation (AF) Ablation
Indice dell'articolo
The state of the art of ablation of atrial fibrillation
The poor success of drug therapy for atrial fibrillation (FA) has encouraged many researchers to explore alternative strategies (1-9). Recent randomized studies have shown that the ablative strategy is superior to therapy with antiarrhythmic drugs in patients with paroxysmal / persistent AF (10-12), and more recently also in patients with “chronic” AF (13). However, further studies are required to confirm these results. In recent years, the number of AF ablation procedures has grown worldwide with increasingly shorter procedure times, thus allowing the inclusion of patients with structural heart disease and long-standing / permanent AF. Due to the excellent success rates reported by the pioneering groups and the attractive possibility of a definitive cure for AF, many patients have started to seek this curative approach, as many electrophysiologists and centers offer it in accordance with the new guidelines. From 1999 to 2007, we carried out more than 15,000 AF ablation procedures in our Electrophysiology Laboratory with a total long-term success rate of > 90% in patients with paroxysmal / persistent AF and 80% in permanent AF, reporting a low incidence of major complications. Despite the development of more and more new technologies and tools, the mechanisms of AF are manifold and many still remain unknown. Three years ago, we demonstrated for the first time the advantage of vagal denervation in patients with paroxysmal AF who underwent ablation and these observations are still a milestone today for understanding the pathophysiology and treatment of AF. Currently, however, we need to have more information on the pathophysiology of permanent AF to measure or limit ablation targets, since patients with long-lasting or permanent AF require extensive ablation and repeat procedures.
Recent data from our laboratory indicate that the progression from the initial paroxysmal form to the persistent or permanent form of AF is relatively rapid and can be predicted by clinical variables (14). As a result, the identification of subjects with a high risk of progression is useful for optimal timing in performing the ablation, avoiding a late procedure when the AF has become permanent. Currently, ablative strategies for patients with permanent AF and associated structural heart disease are complex, long lasting, less effective, and are associated with a high risk of complications. Over the past two years, pioneering groups have confirmed our previous results, even in patients with permanent AF using a gradual approach, which includes the sequential addition of additional targets of ablation through repeated ablative procedures, so as to limit or modify the anatomical substrates, electrophysiological and / or autonomic (15). If the elimination of the substrate is really crucial for the result, the mapping and navigation systems should be able to accurately visualize the complexity of the anatomy of the left atrium, in order to precisely place the lesions, avoiding unnecessary and dangerous radio frequency (RF) applications (Figure 1).
What does circumferential ablation of the pulmonary veins (or CPVA) consist of?
Circumferential ablation of the pulmonary veins (or CPVA) is currently the standard procedure performed in our electrophysiology laboratory. The procedure is performed using manual catheters or remotely with soft magnetic catheters, with faster times than other approaches (16). The CPVA consists of large circumferential lines of injury performed point by point, so as to allow the disconnection of all pulmonary veins (PV), vagal denervation and the non-inducibility of both AF and atrial tachycardia (AT) at the end of the procedure. The data accumulated by our laboratory indicate that, in patients with paroxysmal / persistent AF without atrial magnification, CPVA alone is associated with excellent results, while in patients with long-lasting / persistent or permanent AF and with dilated atria, further linear lesions are necessary to achieve the non-inducibility of arrhythmias.
Objectives of the CPVA
The purpose of transcatheter ablation is to eliminate the trigger and modify the substrate with the least amount of lesions possible. The restoration of the stable sinus rhythm and the non-inducibility at the end of the procedure of both AF and AT is the gold standard of the CPVA. However, many patients with long-lasting / permanent AF, after achieving the sinus rhythm at the end of the procedure, are still susceptible to the induction of sustained AF / AT, requiring additional linear lesions in the left atrium (LA) to achieve non-inducibility. The end points of the standard CPVA procedure include the electrical disconnection of the PVs, vagal denervation, posterior lesion lines and the mitral isthmus line; further linear lesions, including coronary sinus disconnection (CS), are the latest targets. The objectives are achieved with a single mapping / scaler catheter. At present, we do not use the pebble technology since our approach is not limited to disconnecting the PV and since the achievement of multiple objectives precludes its use. In addition, PV have anatomies that vary widely from patient to patient, with a wide range of diameters, and the frequent presence of common ostia in over 30% of patients make it difficult to use this technology.
Disconnection of the pulmonary vein
The circumferential lines are located in the atrial tissue outside the ostia of the PV, an area often called the antrum (Figure 1). The lesions are designed to surround the left and right PVs individually or in pairs. Validation of electrical insulation with the catheter for circular mapping is not performed in our laboratory, since we have performed a real distal electrical insulation through the reduction of potential reduction (reduction > 90% of the electrogram amplitude), even within the surrounded areas (electrogram amplitude < 0.1 mV). Disconnection of the PVs is obtained with the optimal stability of the catheter and the contact with the wall that remains in a rapid attenuation of the atrial electrograms during each delivery of RF until their complete elimination (Figure 2).
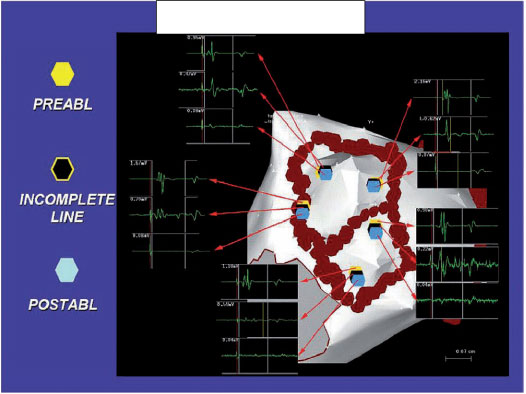
Signals in partially ablated areas require additional RF applications before moving on to the next ablation site.
Autonomic objectives of vagal denervation
Whenever possible, the elimination of vagal reflexes in innervation sites during the procedure is one of the most important objectives, since vagal denervation is a strong predictor for the long-term success of the ablation procedure (Figure 3). We first demonstrated that CPVA induces not long-term, but transient, vagal denervation, which in any case increases its long-term efficacy (8). These results have been confirmed by many other authors with different ablative approaches, and now vagal denervation constitutes a fascinating new strategy for AF ablation. Our results on changes in HRV after ablation add new insights for understanding the mechanisms of AF and its treatment. While standard CPVA lesions are practiced, RF deliveries evoke vagal reflexes in approximately 30% of patients. Sinus bradycardia (HR < 40 beats per minute), asystole, AV block, and hypotension occurring within seconds of the start of RF application should be counted as vagal reflexes (Figure 3).
If a reflex of this type is evoked, RF energy is supplied until such reflexes are abolished, or up to a maximum of 30 seconds. The purpose of ablation in these sites is the cessation of the reflex, followed by sinus tachycardia or AF. Failure to reproduce reflexes with repeated RF applications is considered a confirmation of denervation. Based on our experience, we have always tried to stimulate, and therefore ablate, these sites for vagal denervation. We have reported a detailed “autonomic map” of the left atrium as an ablation target, showing that like the left upper pulmonary vein, the septal region is also richly innervated (8).
Ablation of the posterior line and mitral isthmus
In the standard CPVA procedure, additional ablation lines are practiced along the posterior wall and the roof of the LA between the two series of lesions that connect the upper and lower PVs and the mitral annulus (Figure 1). The mitral isthmus line is used to prevent post-ablation atrial tachycardia (5,16,17) and to further reduce the arrhythmic substrate (Figure 3). The completeness of the mitral isthmus line is an important electrophysiological goal and is validated during epicardial stimulation from the coronary sinus (CS) and during mapping of the coronary sinus, looking for double potentials along the block line, and confirmed by differential pacing (5). The minimum interval between double potentials in the mitral isthmus during pacing from CS after block is about 150 ms, depending on the atrial size and the extent of the scars and lesions (5).
Ablation of the tricuspid hollow isthmus line
Patients with AF and a history of common atrial flutter or patients with permanent AF undergo ablation of the cavotricuspidal isthmus line. If all endpoints are reached at the end of the standard CPVA and the patient is in sinus rhythm, the non-inducibility of AF / AT is the ultimate goal.
Additional linear lesions and disconnection of the coronary sinus
If the inducibility of AF and AT persists even after cardioversion, we carefully review the lesion lines and the ablated areas to verify potential residues and apply radio frequency when needed. If necessary, additional ablation lines are performed (usually roof, septum or LA base) before isolation of the CS, which is the ultimate goal (Figure 4).
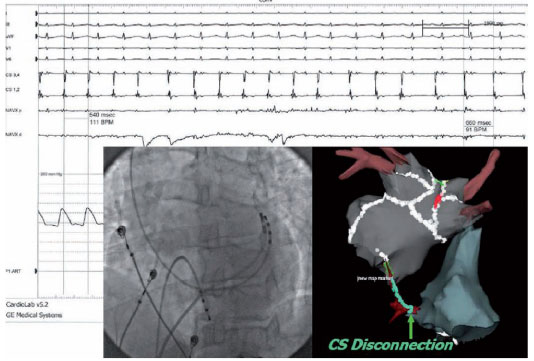
The compartmentalization is assessed by the presence of a “corridor” of double potentials and by the demonstration of activation towards the block line on both sides. A complete LA roof line can be demonstrated by progressive caudocranial activation on the posterior wall during LA stimulation. Fervent atrial activity from the CS musculature can be a conductor for long-lasting or permanent AF. The electrical disconnection of the coronary sinus from the atrium is performed with ablation at the endocardial or epicardial level (or both). The total elimination of the electrical activity of the coronary sinus is the ideal goal, but the organization of the electrical activity of the CS and / or the slowing down of the local frequency with dissociation between the activity potential of the CS and LA is also considered as proof of CS isolation. Endocardial and / or epicardial CS sites are frequent ablation targets in patients with permanent AF and dilated atria.
Post-ablation remapping
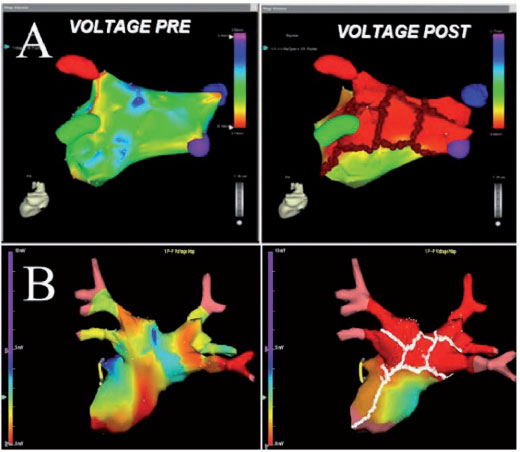
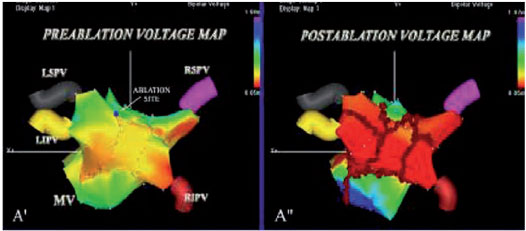
Once the non-inducibility of the AF / AT has been obtained, the LA is re-mapped, and the pre-ablation and post-ablation activation maps are compared (Figure 1). In patients in sinus rhythm, LA post-ablation remapping is done using the pre-ablation map for the acquisition of new points to compare the pre- and post-ablation bipolar voltage maps. In AF patients, after restoration of the sinus rhythm, post-ablation mapping is carried out using the anatomical map acquired during AF to validate the accuracy of the lesions. The incomplete block is revealed by the propagation of the impulse throughout the ablative line and requires further RF applications to complete the line despite the non-inducibility.
What are the patient selection criteria for the atrial fibrillation ablative procedure?
In recent years, the indications for the ablation of AF through CPVA have expanded widely, based on the results of numerous clinical trials. The procedure is primarily indicated in symptomatic patients with atrial fibrillation refractory to antiarrhythmic drug therapy. Currently the procedure is recommended at an early stage of the disease, independently of the refractoriness of antiarrhythmic drugs. In recent years, the indication has also extended to patients with heart failure and valvulopathies, and also in older subjects, in subjects with permanent AF and / or mitral or aortic mechanical valve prostheses. A low ejection fraction of the left ventricle does not represent an absolute contraindication to CPVA. Indeed studies, such as CASTLE-AF, have shown that these patients also benefit from ablation. Recent studies have highlighted the usefulness of continuing antiarrhythmic therapy after ablation. Therefore therapy with antiarrhythmic drugs is generally continued after ablation, and dosages are generally progressively reduced. Anticoagulant therapy is also generally continued after ablation, and is suspended on a case-by-case basis after AF ablation.
Pre-procedure preparation
The transesophageal echocardiogram (TEE) is the examination of choice to exclude the presence of thrombi in the left atrium or auricle, which are considered an absolute contraindication to the ablative procedure, which is postponed until the presence of cardiac thrombi is excluded from a new TEE during anticoagulant therapy (both with DOAC and Dicumarolic drugs). At discharge, a transthoracic echocardiogram is usually performed. Three days before the procedure, patients taking oral anticoagulant therapy stop it. The night before ablation, heparin infusion is started to reach ACT values between 200 and 250 seconds; heparin is stopped only 2 hours before the procedure to safely perform transseptal puncture. A weight-dependent infusion dose of narcotic, such as remifentanil (0.025-0.05 mcg / kg / minute) is also used. Cardiac surgery is readily accessible to perform emergency surgical procedures when needed. An echocardiography in the electrophysiology laboratory is available primarily for the diagnosis of pericardial tamponade.
The transseptal puncture
Usually, before the transseptal puncture, a catheter is inserted into the coronary sinus to map the left atrial activity, and a multipolar catheter is placed in the right atrium to map the electrical activity of the right atrium. CPVA requires a single transseptal puncture for the mapping / ablation catheter. After transseptal access, a single bolus of heparin is administered intravenously, and two blood samples are taken every 15 minutes to check the ACT, which must be maintained > 250 s or more.
Identification of ablation targets
An accurate identification of the targets and a relatively short ablation are required to avoid major complications and successfully achieve all the objectives. Currently, this is facilitated by the use of 3-D navigation and mapping systems that provide precise orientation from the anatomical and electrophysiological point of view. CPVA is performed in about 1 hour, but it can be longer (up to 3 hours) in patients with permanent AF with dilated atria to achieve all objectives including disconnection of CS and non-inducibility of AF / AT. We do not routinely use intracardiac echocardiography and the Lasso catheter.
Electroanatomical mapping
Usually in our Electrophysiology laboratory, we use the CARTO mapping systems (BiosenseWebster, Diamond Bar, CA, USA) and the EnSiteNavX (St. Jude Medical, St. Paul, MN, USA), which have significantly shortened the time of fluoroscopy, improving the safety profile of the procedure (figure 1). The early adoption by our group of the CARTO mapping system has allowed an accurate reconstruction of the complex left atrial anatomy and is now accepted by the entire electrophysiology community that performs AF ablation (2). The CARTO system continuously locates the position of the catheter using three very low magnetic fields, while the NavX system is based on electric fields generated by three pairs of skin electrodes orthogonal in three axes: X, Y, and Z. (Figure 1). Unlike the CARTO, the new NavX allows to obtain a 3-D reconstruction of both the tip and the body of the catheter, which is particularly useful in “difficult” areas, such as the ostia of the PV, the crest, the mitral annulus and the septal area. The monitoring of the catheter with the NavX system is obtained by a proximity indicator which, based on the color intensity of the tip of the catheter, allows the operator to check the optimal contact of the ablation catheter, a contact that when associated with the abatement of the atrial potential indicates achievement of the goal (Figure 1). During RF applications, cardiac movement, pain and breathing are all factors that affect the stability of the positioning of the catheter, but NavX software allows to minimize the amount of target movement, as well as respiratory artifacts. When the posterior wall is ablated, which is a vulnerable area at greater risk of cardiac perforation, the presence of pain can cause changes in the respiratory rate, and respiratory compensation by the NavX is useful for maintaining catheter stability. Furthermore, Navx technology is able to create separately any desired anatomy for each ablation target which results in a more accurate ablation, in particular of difficult targets, such as the ostia of the PVs, their cavern, the posterior wall, and the CS (figures 4 and 5).
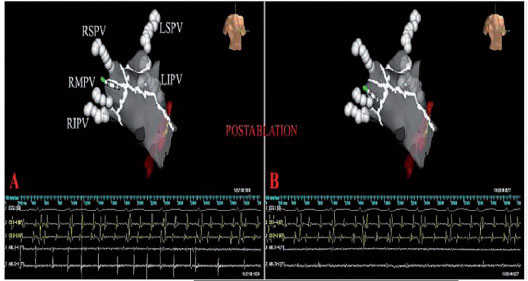
Although the NavX Ensite system allows you to collect many points quickly and sequentially, in difficult areas, it is preferred to acquire points manually as in the CARTO system. Another important advantage of the NavX system, compared to the CARTO, is that the patient’s movements during the procedure do not concern the reconstruction of the map, as the reference catheter also moves due to the presence of patches attached to the patient’s body. As for the CARTO system, after ablation, a voltage map is shown by a colorimetric gradient to verify the complete elimination of potentials along and within the lesion lines (Figure 1). Currently, with both electroanatomical systems, in a few minutes we are able to reconstruct the anatomy of the LA and the ablation targets. The reconstruction of the PVs and their hosts represents the first step and is confirmed by the simultaneous use of fluoroscopy, electrograms, and impedance gradients. Typically and simultaneously, once the catheter enters the PV, the tip is seen outside the heart shadow on fluoroscopy, the impedance values significantly increase (over 4 Ohms above the left atrial impedance), and the atrial electrograms disappear. Once the PVs are displayed, a detailed sequential reconstruction of the left atrium is performed, including the rear and front walls, the LAA, the roof, the septum and the mitral annulus with its isthmus. The septum and the channel between LAA and the LSPV often require the acquisition of many more points than in other areas. LAA, which is identified with the presence of unfractionated and large amplitude atrial electrograms and large ventricular electrograms with an electrical activity organized in AF, is one of the last areas that is mapped. The channel between LAA and LSPV shows potentials that are typically smaller than those of LAA but higher and more fractionated than in the rest of the left atrium. If the canal is not accurately rebuilt, the left side of the circumferential lesion can be positioned too close to the LAA or within the PV ostium, which can result in poor efficacy and major complications, such as perforation of the LAA stenosis of the PV. Although roof reconstruction is easier by requiring fewer points to acquire, incorrect interpolation of the roofs should be avoided when using the CARTO system.
Ablation of desired targets
Once the left atrium and the main pulmonary veins have been adequately reconstructed, radio frequency energy is supplied, which in our laboratory is the most frequently used type of energy, for the endocardial ablation of the aforementioned electrophysiological and anatomical targets. Over the past three years, we have used an irrigated 4 mm catheter instead of the irrigated 8 mm, which has been shown to have some limitations, including the propensity for clot formation and insufficient energy delivery in areas with low blood flow. The irrigated catheter allows to adequately distribute the energy and to obtain larger lesions, minimizing the embolic risk. In our approach, the effectiveness of radio frequency delivery is and remains important, but we try to moderate the power in risk areas for greater safety. We usually use a lower power setting (30-50 W) and an irrigation flow of 2 ml/min (during mapping) and up to 50 ml/min during ablation (based on the site of delivery of the radio frequencies). For circumferential lesions, radiofrequencies are delivered at a distance of about 1 cm from the ostia (instead of 5 mm), thus reducing the risk of stenosis of the pulmonary veins. If an increase in impedance occurs (> 10 Ohms) or the patient experiences burning pain, the radio frequencies are stopped immediately. When the ablation starts, the irrigation flow increases from 2 to 17 ml/min, while the impedance and temperature values at the tip of the catheter are constantly monitored. The output energy is limited to 50 W with a maximum temperature of 48 degrees C throughout the procedure, but lower values are used in the posterior wall and in the coronary sinus to reduce the risk of injury to adjacent structures. Usually the circumferential lesion lines are practiced starting from the lateral portion of the tricuspid annulus and moving posteriorly, then anteriorly to the left of the pulmonary veins, passing the ridge between the LSPV and the atrium and going close to the lesion on the posterior wall of the atrium. The right pulmonary veins are isolated in a similar way, and two further lines connecting the two circumferential lines are made posteriorly. The circumferential lines are adapted according to the individual anatomy of the junction between the pulmonary vein and the atrium. A single circumferential line surrounds the two ipsilateral VPs in the presence of ostia less than 20 mm apart, in the presence of a common ostium or an early branch division. If anatomically possible, we also practice a line of injury between the two ostia to further reduce the anatomical and electrophysiological substrate. Characteristically, in patients with permanent AF and dilated atria, while performing the disconnection of the coronary sinus and before restoring the sinus rhythm, there is a regularization of the cycle with a transformation in CT and a uniform morphology of the P wave. With our approach, there is a restoration of the sinus rhythm (SR) in almost all patients with permanent AF. The restoration to SR occurs immediately or after transformation into AT. The procedure is successful when all endpoints are reached.
Ablation of critical areas
Obtaining all endpoints is crucial but can be difficult in specific areas. Repeated applications of short duration radio frequency, high intensity and higher irrigation flow are usually needed around the VPSS, where atrial potential is difficult to eliminate. The complete removal of atrial potentials in the ridge between the VPSS and the auricular requires longer RF applications with higher power. If the ridge is too narrow, the ablation line is made by passing to the base of the auricle. The VPDs and the mitral isthmus are two other difficult sites for both mapping and ablation and require constant adjustments in the RF setting. Incomplete lesion lines, especially in the vicinity of the mitral isthmus, can remain in gaps that support an incessant post-ablation atrial tachycardia. In patients with mechanical valve prostheses, mapping and ablation near the mitral area can be difficult; however, no experience of catheter entrapment has occurred in our experience. The mitral isthmus line requires validation of the disconnection with pacing maneuvers and in a minority of patients also applications within the coronary sinus. The ablation of the connection sites between the SC and the atrial musculature requires a lot of attention and requires lower settings in the energy and irrigation flow to avoid perforation and cardiac tamponade. Usually we practice two low energy radio frequency applications (between 15 and 30 W) from the distal to the proximal, instead of a single application, to keep the temperature low and avoid potential complications. The posterior wall also represents an area potentially at risk of complications, such as atro-esophageal fistula and cardiac tamponade. It is well known that the posterior wall is not only the thinnest wall of the left atrium, but is in close correlation with the esophagus. When we apply radio frequencies in this area, we use a lower setting in terms of energy and irrigation flow.
Management post-procedure and of complications
At the end of the procedure, we usually use protamine sulphate to allow the removal of the introducers. Subsequently, management includes anticoagulant therapy, while in the past embrication with heparin was used. Currently with DOAC drugs, this is no longer necessary and only oral anticoagulant is continued. The possibility of optimizing the parameters according to the most critical areas allows for a lower incidence rate of major complications. Cardiac tamponade should be excluded in all patients who have post-procedure hypotension. In our experience, however, this complication is very rare if you pay attention to the settings used. Only a few patients required pericardiocentesis following pericardial effusion, and we reported only one case of atrioesophageal fistula. The late onset (6-10 days after ablation) of a febrile state with or without neurological symptoms should always lead to suspicion of esophageal atrium fistula, which should be excluded by means of a spiral CT with contrast. In our extensive experience covering over 15,000 cases of CPVA, there have been no perioperative deaths, or major complications, such as VP stenosis, phrenic nerve injury or coronary artery occlusion. Minor complications are infrequent, while a non-hemodynamically significant pericardial effusion affects about 4% of patients. Pericarditic pain may be present in the early days of the post-procedure and is usually responsive to salicylates.
Post PTCA rhythm control
The absence of symptoms may not correspond to a stable restoration of the sinus rhythm, and the accuracy of the evaluation of post-ablation recurrences most of the time depends on the duration of the ECG recordings. To assess what the burden of asymptomatic recurrences of arrhythmia is, usually after ablation, patients undergo a loop recorder implant (generally within 45 days of ablation), followed by remote monitoring. Alternatively, patients can undergo Holter ECG recordings after 1, 3, 6 and 12 months and a transtelephonic ECG (cardiotelephone) monitoring.
Effectiveness
In the first two months after the procedure, atrial fibrillation recurrences may occur, however in half of the cases they constitute a transient phenomenon and do not require a second procedure. The long-term efficacy of CPVA is > 90% in patients with paroxysmal atrial fibrillation, and approximately 85% in patients with permanent atrial fibrillation, when it was not possible to induce AF or AT at the end of the procedure. The long-term success rate is higher in patients with paroxysmal atrial fibrillation and local vagal denervation. If there is a recurrence of persistent atrial fibrillation or frequent episodes of symptomatic atrial fibrillation or the presence of a symptomatic right or left atrial flutter, a second procedure is proposed at least six months after the first. The procedure is repeatable for a maximum of three times.
Atrial remodeling
The assessment of the potential consequences of ablation on the contractility of the atrium is important for the correlation of this with the thromboembolic risk. After ablation, we carefully evaluate the contractile function of the left atrium, both in the immediate post-procedure and during long-term follow-up. In our experience, after ablation, the diameters of the left atrium are reduced and the contractile function improves, but the significance of these improvements depends strictly on the atrial dimensions before ablation. In patients without relapses and with good atrial function, we stop anticoagulant therapy.
Post-ablation atrial tachycardia
If all objectives have been achieved during the procedure, post ablation atrial tachycardia develops in less than 5% of cases, and it is usually macro / micro re-entry tachycardia, rather than focal atrial tachycardia. In our experience, these tachycardias should initially be treated conservatively through drug therapy or cardioversion. Only in symptomatic patients is the procedure repeated in order to optimize ablative therapy, and in many cases therapeutic success is achieved. Ablation should be performed not through empirical lesions but after recognition of the underlying mechanism. The morphology of the P wave, its axis, and the continuous activation of the atrium leads to a macro-reorientation mechanism, while the observation of an isoelectric line between the P waves leads to a focal tachycardia. We routinely perform both a voltage and activation map; combining them together with pacing maneuvers for a better result of ablative therapy. Usually the activation map shows the earlier and later activation with a chromatic scale that refers to a time window equal to the tachycardia cycle. The most common post-ablation atrial tachycardia is due to one originating from the mitral annulus. Entering with post-pacing intervals equal to the tachycardia cycle measured at more than three sites around the upper and lower mitral annulus, with an activation time around the tricuspid annulus equal to the tachycardia cycle, strongly suggest a diagnosis of atrial tachycardia originating from the mitral annulus. As in the case of the isthmus-dependent right atrial flutter, the narrowest area of the circuit is located between the VPIS and the annulus. Consequently, the best place to look for the residual gaps and to repeat the ablation is the mitral isthmus. For micro reentrant atrial tachycardias (cycle length less than 80%) originating from the reconnection of the VP ostia, the ablation of the sites with earlier activation that have an occult entrainment proves to be very effective. Frequently the voltage maps show areas of voltage preserved at the early activation sites, suggesting the presence of areas not previously ablated or insufficiently ablated. The re-entry around the right or left pulmonary veins can be demonstrated by pacing from the distal and proximal coronary sinus, from the septum and from the roof of the atrium. Their management requires the use of 3-D activation maps to outline the course of tachycardia and to identify a lesion line that connects the anatomical barriers in order to interrupt the atrial tachycardia circuits. RFs are delivered after having clearly identified the critical isthmuses with a detailed electroanatomical map. Usually only a few RF applications are needed to eliminate tachycardia circuits and their inducibility.
Anticoagulation
Stroke is a possible and feared complication of AF ablation, in particular if one considers the possibility that there may be asymptomatic ischemic episodes. To prevent stroke or other embolic thrombus events in patients who are not chronically taking anticoagulant therapy, a trans-esophageal echocardiogram is performed after short-term anticoagulant therapy, instead of after three weeks. Pre-ablation anticoagulation patients with permanent atrial fibrillation, the patients at risk (patients with persistent atrial fibrillation or with paroxysmal atrial fibrillation associated with other risk factors), require oral anticoagulation therapy for at least three weeks documented by a careful monitoring of the value of INR. We also recommend performing a TEE before the procedure in all patients who have AF or who have a high risk of thrombotic events.
Anticoagulant therapy during the ablation procedure
Anticoagulation should be performed after performing the transseptal puncture and it is often necessary to maintain an ACT value > 300 sec to reduce the risk of thrombosis of the introducer. What is the best protocol for anticoagulation in the post-procedure has not yet been established. Due to the embolic risk in the post-procedure, the patient is suggested to observe oral anticoagulant therapy in the first 3-4 months. In selected patients who have no evidence of arrhythmic episodes 4-6 months after ablation, we stop coumadin and set up aspirin therapy (75-325 mg/day). In any case, patients with high embolic risk should continue warfarin therapy even if there is no evidence of arrhythmic recurrences.
Remote mapping and ablation with Stereotaxis
Currently most of the transcatheter ablation procedures are performed manually in a traditional way, and this requires qualified and experienced personnel in the handling of the catheters and in the ablation. In a modern electrophysiology laboratory, the presence of magnetic navigation systems means that the differences due to the human factor are limited, and the results are more reproducible. The feasibility of a remote system that is not an employee operator, could represent an interesting and attractive alternative for laboratories, which could thus obtain a high success rate while minimizing risks. The recent possibility of having a magnetic catheter with an irrigated tip will increase the benefits of the remote system by being able to perform deeper procedures regardless of the operator’s experience. We have shown that remote navigation could facilitate both mapping and aeration regardless of the dexterity of the electrophysiologist. The magnetic navigation system uses soft catheters equipped with three small magnets on the tip for optimal orientation in the magnetic field created by two large magnets positioned on both sides of the operating table. This system consists of two independent components that communicate with each other: the Niobe Stereotaxis MNS and the electro-anatomical mapping system CARTO-RMT. The Niobe includes a computer interface that is controlled by a keyboard and a joystick that changes the orientation of the two magnets by changing the orientation of the magnetic field and therefore the location and orientation of the tip of the catheter. The operator is in a separate room, away from fluoroscopy and the patient’s body. This system is combined with the CARTO mapping system which has been modified to support magnetic navigation. A 4-8 mm magnetic tip catheter (Navistar-RMT, Biosense Webster, Inc.) can be connected to the CARTO-RMT, and irrigated tip catheters are also available in Europe. The three magnets present in the distal portion of the catheter allow the catheter to have wide orientation possibilities, while the movement is guaranteed by a mechanical device (Cardiodrive Stereotaxis). The magnetic field vectors used for each navigation and ablation target can be stored and reused for automated ablation. An accurate electroanatomical map can be created simply by using the automatic function present in the Navigant software, which has been specifically designed for the mapping of the left atrium. There is also the possibility of taking additional points in areas of particular interest. The sequential acquisition of different points all around the left atrium with a stable wall contact of the lead allows to accurately recreate the cardiac geometries of even the most complex areas with a surprising degree of accuracy and effectiveness. In our experience, remote mapping and ablation was possible in all patients who underwent atrial fibrillation ablation. Initially, the procedure times were a little longer than manual procedures, and this was due to the learning curve, which, in the early stages, requires frequent adjustments in the orientation of the catheter tip. The ablation times to complete the lesion lines around critical areas, such as the right VP, are shorter remotely than with manual ablation, suggesting that, with magnetic navigation, there are no sites difficult to ablate, thus avoiding unnecessary RF applications and consequently major complications.
Advantages of the remote system
The orientation of the lead is entirely guided by the magnetic field vectors, and also the RMT catheter is much softer than traditional catheters near the distal segment. If the catheter does not reach the predetermined location, the operator must simply move the catheter from the anatomical obstacle and advance to the desired location by manipulating the magnetic field. This leads to a lower traumatization of the endocardium and a lower risk of cardiac perforation. Also in our experience, no cases of cardiac perforation have been reported during the mapping of the areas of the left atrium with thin muscle walls. The “soft touch” catheters used in magnetic navigation lead to a lower deformation of the heart chambers compared to traditional catheters, and this to the advantage of a more accurate anatomical reconstruction and less use of fluoroscopy.
Limitations of the remote system
There are some limitations of the system that can be resolved as technological progress advances. The size and position of the magnets can interfere with the fluoroscopic vision of the heart during the procedure. However, this drawback can be overcome by the presence of a more accurate electroanatomical map.
