Wireless Pacemaker
Indice dell'articolo
What are leadless pacemakers?
With the evolution of technology, “wireless” leadless pacemakers have been developed, in which the pulse generator and the electrodes are contained in a single intracardiac unit, thus eliminating the presence of the conventional leads and subcutaneous prepectoral pocket.
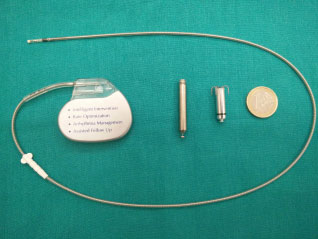
So far, two types of leadless pacemakers have been developed for implantation in humans: Nanostim (from St. Jude Medical) and Micra (from Medtronic). The two devices differ in size: the Micra is shorter but with a slightly larger diameter (25.9 x 6.7 mm) than the Nanostim (42 x 5.99 mm) (figure 1). The devices also differ in the fixing system to the wall of the right ventricle: the Micra has a fixing system to the heart muscle consisting of 4 self-expanding wires, while the Nanostim has an active fixing system consisting of a screw that must be inserted in the thickness of the heart muscle. The longevity of the battery is similar to that of traditional pacemakers (7-10 years). The Nanostim device has been temporarily withdrawn from the market due to problems related to the coupling system, therefore only the Micra device is currently available.
What are the advantages of leadless pacemakers?
The traditional pacemaker implantation presents critical issues, both in the operating phase and in the subsequent follow-up, given that there is a need for a surgical approach with incision of the skin and consequent scar development. In the acute phase, complications such as pneumothorax, cardiac perforation, dislocation of catheters, and hematoma of the pocket are possible, albeit infrequently. Local follow-up complications related to the presence of the subcutaneous generator and leads can occur, such as local or systemic infections with the potential for bacterial endocarditis, venous occlusion or tricuspid valve failure. This generally implies the need for explantation and complete extraction of the stimulation system, a procedure not without complications.
The advantages of leadless pacemakers are essentially related to their small size, minimum weight, the absence of connection mechanisms between the generator and electrodes (since they coexist in a single unit), the minimally invasive transcatheter implant procedure, and the much lower risk of infections.
Among the advantages, there is also the positive psychological impact linked to the absence of scar (since there is no need for surgical incision) and subcutaneous pocket. For the Micra device, the only leadless pacemaker currently in use, compatibility with total body MRI is also possible.
What are the limitations of leadless pacemakers?
Wireless cardiac pacing, however, still has some limitations related to the fact that, so far, only devices suitable for single-chamber stimulation are available (for now only right ventricular). Therefore, these systems are not suitable for patients who need dual chamber stimulation or resynchronization.
Furthermore, further long-term data are still needed to define the performance of the fixing mechanisms and the feasibility of extracting the devices.
What are the current indications of leadless pacemakers?
The current indications for leadless pacemakers therefore concern, at the moment, only a relatively small category of patients with the need for single chamber stimulation, such as patients with permanent ventricular slow atrial fibrillation, or in some cases, of paroxysmal atrioventricular block, or patients with a history of secondary infections of intracardiac devices.
How is the leadless pacemaker implantation performed?
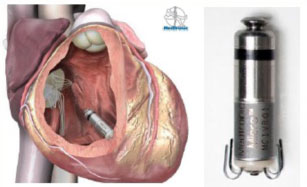
The leadless pacemakers are positioned in the right ventricular cavity with a minimally invasive procedure via the catheter with a transcutaneous approach using the femoral vein, through a special introducer. The system therefore does not require the creation of a pocket and leaves no visible external scars.
