Implantable Loop Recorder
Indice dell'articolo
What is the “Implantable Loop Recorder” (ILR)
The implantable loop recorder (ILR), also known as an injectable cardiac monitor (ICM) is a subcutaneous device used to diagnose heart rhythm disorders. These devices, available since the 1990s, have been improved and miniaturized over the past few years, and have become essential tools for the diagnosis of infrequent heart arrhythmias. The recent advent of injectable ILRs makes the procedure even simpler and more tolerated by patients. The loop recorder records and stores the ECG traces and can be interrogated remotely, i.e. without patient access to the facility.
What is the “Implantable Loop Recorder” (ILR) for?
The Implantable Loop recorder (ILR) is mainly used to document infrequent arrhythmic events in various clinical conditions, particularly in unexplained syncopes and palpitations. The latest generation ILRs are equipped with sophisticated algorithms capable of detecting episodes of atrial fibrillation. This new opportunity may provide physicians with systematic heart rhythm screening with possible effects on the management of patients’ anti-arrhythmic and anticoagulant therapy, after transcatheter ablation or after cryptogenic stroke.
In patients with unexplained recurrent syncope (with uncertain diagnosis) the ILR has proven to be the instrument with the highest diagnostic efficacy, since it reveals the relationship between events and cardiac arrhythmias, in particular the presence of slowing of the heart rhythm, or bradyarrhythmias, or pathological acceleration of the heart rhythm (supraventricular or ventricular tachyarrhythmias).
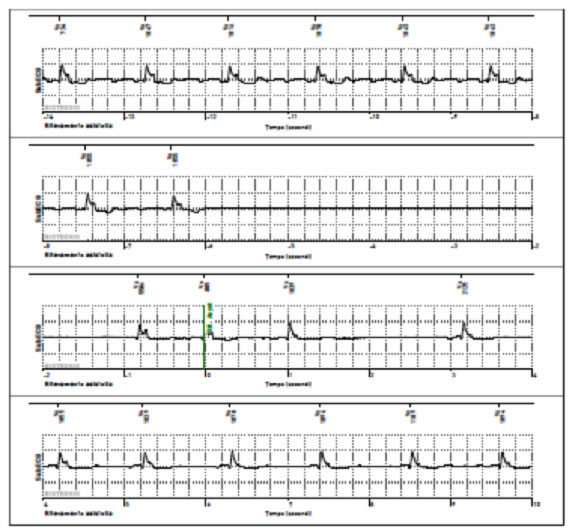 Example of bradyarrhythmia with symptomatic pause> 6 sec for presyncope in patient with ILR
Example of bradyarrhythmia with symptomatic pause> 6 sec for presyncope in patient with ILR
- In patients with arrhythmic heart disease of unexplained origin, the ILR can identify episodes of tachyarrhythmia, due to paroxysmal supraventricular tachycardias, atrial fibrillation or more rarely, ventricular tachycardias.
- In patients following atrial fibrillation or flutter catheter ablation procedures, the ILR is used to evaluate the burden of atrial fibrillation, to evaluate the indication to continue anticoagulation therapy and antiarrhythmic therapy, or any need of a new ablation or electrical cardioversion intervention.
In patients with cryptogenic stroke (of uncertain origin) the ILR is used to look for episodes of silent paroxysmal atrial fibrillation, and to evaluate the indication for anticoagulant therapy, antiarrhythmic therapy, or transcatheter ablation for atrial fibrillation.
How does the Implantable Loop Recorder (ILR) work?
The Implantable Loop Recorder (ILR) is based on the recording of electrocardiographic traces by means of a dipole obtained through two electrodes inserted directly into the device case. ILR recorders are equipped with a retrospective memory (loop recorder memory), through which they record the ECG tracing continuously and store only a few recording segments afterwards. The storage takes place both in case of symptoms, when the patient manually activates the recorder by means of a special remote control, and automatically, if the device detects the presence of predefined arrhythmias. The duration of the recorded ECG traces varies according to the devices, up to a maximum of 30 minutes for the symptomatic episodes in the latest generation devices.
The internal storage capacity of the ECG traces is also variable depending on the devices: once this capacity is exhausted, in order to store new traces, the older traces are gradually deleted, and only a short text file remains available. This is the reason why ILRs are generally followed with remote monitoring.
ILR models produced by three different companies are currently available (Biotronic, Medtronic, Saint Jude Medical): with technological evolution, the size and weight of ILRs have gradually decreased, becoming similar or smaller than a package of chewing-gum, while the storage and duration of ECG monitoring have progressively increased, given that current systems are able to monitor the heart rhythm for up to 3 years.
How is the implantation (insertion) of the Implantable Loop Recorder (ILR) performed?
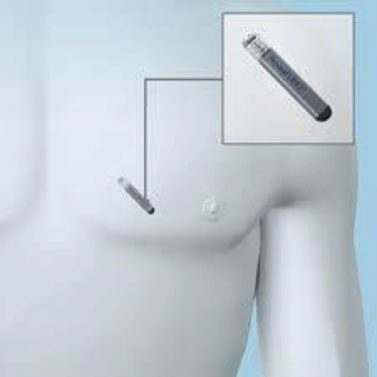
The Implantable Loop Recorder is either inserted into the subcutaneous tissue by creating a small surgical pocket, or, in the more recent models, the recorder is inserted through a special subcutaneous insertion system (injector) that creates a minimal skin breach without the need for surgical preparation of a pocket. Normally the implant takes place under local anesthesia, after a short hospitalization, in the precordial region, and there are no particular limitations in the days following the implant.
What are the complications of the Implantable Loop recorder system?
The complications of the implant are very rare, and are generally limited to mild pain or a small hematoma in the implant area, which regresses after a few days with mild pain-relieving drugs. In very rare cases where an infection or an important hematoma of the pocket occurs, the device may be explanted, which takes place through a small surgical incision, always under local anesthesia, without further general consequences.
How is the Implantable Loop Recorder information accessed?
The information access of ILRs can take place in the clinic through the classic programmers that are used to check implantable cardiac devices (pacemakers and defibrillators).
The information access for ILRs, as for other implantable cardiac devices, can however take place with remote monitoring, through a special transmission monitor that is delivered to the patient. In the case of ILRs, remote monitoring has a particularly important and indispensable function, since the remote information gathering from ILRs not only avoids unnecessary patient access to the hospital structure, as well as earlier diagnosis of any arrhythmic events, but it also prevents the saturation of the memory of the devices, significantly increasing the diagnostic efficacy.

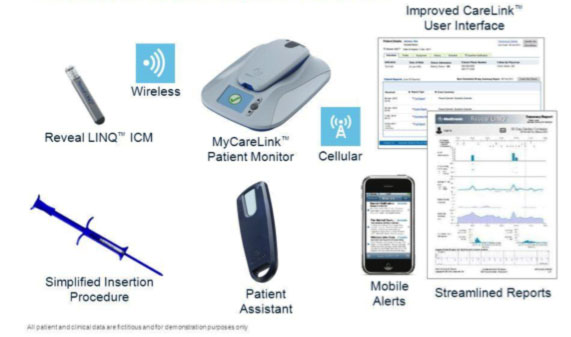
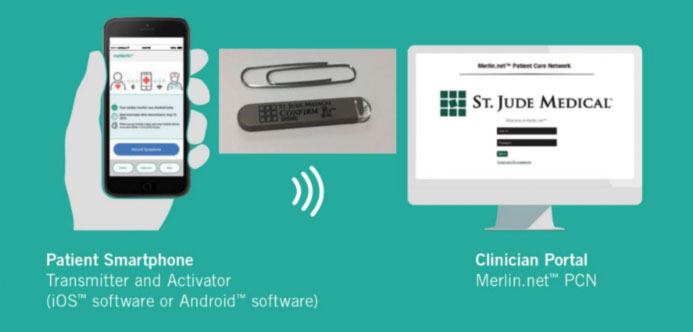
What is our experience with ILR?
In our center, we have a long period of experience with ILR implants, which are used for the diagnosis of syncopal or presyncopal episodes of unexplained origin, the diagnosis of episodes of arrhythmic heart rate, and the monitoring of arrhythmic burden after transcatheter ablation of atrial fibrillation or tachycardias with or without accessory pathways, and finally to check for embolisms in atrial fibrillations in patients with cryptogenic stroke.
To date, over 2000 ILRs of the three manufacturing companies have been implanted in our center, and all devices are regularly monitored with remote monitoring.
